

Now, the gas thermometers can be used to define it in a certain way. Absolute zero temperature has zero magnitudes on a Kelvin scale. Helium and hydrogen are the most widely used in gas thermometers. What is the constant volume?Īn isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. The triple point occurs at a fixed temperature and pressure for a specified substance. Ideal gas temperature scale can be developed by measuring the pressures of the gas in the vessel at two reproducible points (such as the ice and steam points) and assigning suitable values to temperatures those two points. They are simple, inexpensive, long-lasting, and able to measure a wide temperature span. Liquid thermometers are the most common type in use. Gas thermometers work best at verylow temperatures. What is the importance of gas thermometer? a constant pressure process is said to be isobaric. This relationship is known as Charles’ law or Gay-Lussac’s law. The ratio of volume to temperature is constant when pressure is constant. The volume of a gas is directly proportional to its temperature when pressure is constant. Gas Thermometer Determinations of the Thermodynamic Temperature Scale in the Range -183 ☌ to 100 ☌. (iii) It cannot measure accurately the rapidly changing temperatures. What is the limitation of gas thermometer?ĭisadvantages of gas thermometers: (i) It is not a direct reading thermometer. In 1702, Guillaume Amontons (1663–1705) invented the gas thermometer. Who invented constant volume gas thermometer?Ģ.5 A first look at gas thermometry So, although satisfactory for everyday applications, the mercury-in-glass thermometer is not suitable for wide–ranging scientific work. Gas thermometer can be used for wide range of temperature. The expansion coefficient of all gases is nearly the same, so thermometer using different gases give same reading. What are the advantages of constant volume gas thermometer?Īdvantages of constant volume Gas thermometer:-) The gas expand uniformly and regularly over a wide range of temperature.
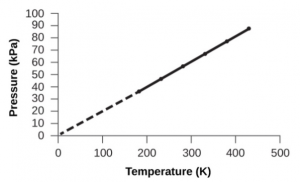
It is seldom used as everyday laboratory thermometer because it is not easily handled unlike the laboratory thermometer. (ii) The constant – volume gas thermometer measures temperature more accurately than other thermometers because its glass expansion is negligible compared to the expansion of the gas. Why is a constant volume gas thermometer seldom used? Note: Charle’s law can also be defined as when the pressure remains constant, then the volume of the gas increases or decreases by the value 1273.15 of its volume at 00C for each 10C rise or fall of temperature. How does a constant volume thermometer work?Ī constant volume gas thermometer works on the principle of Charle’s law.
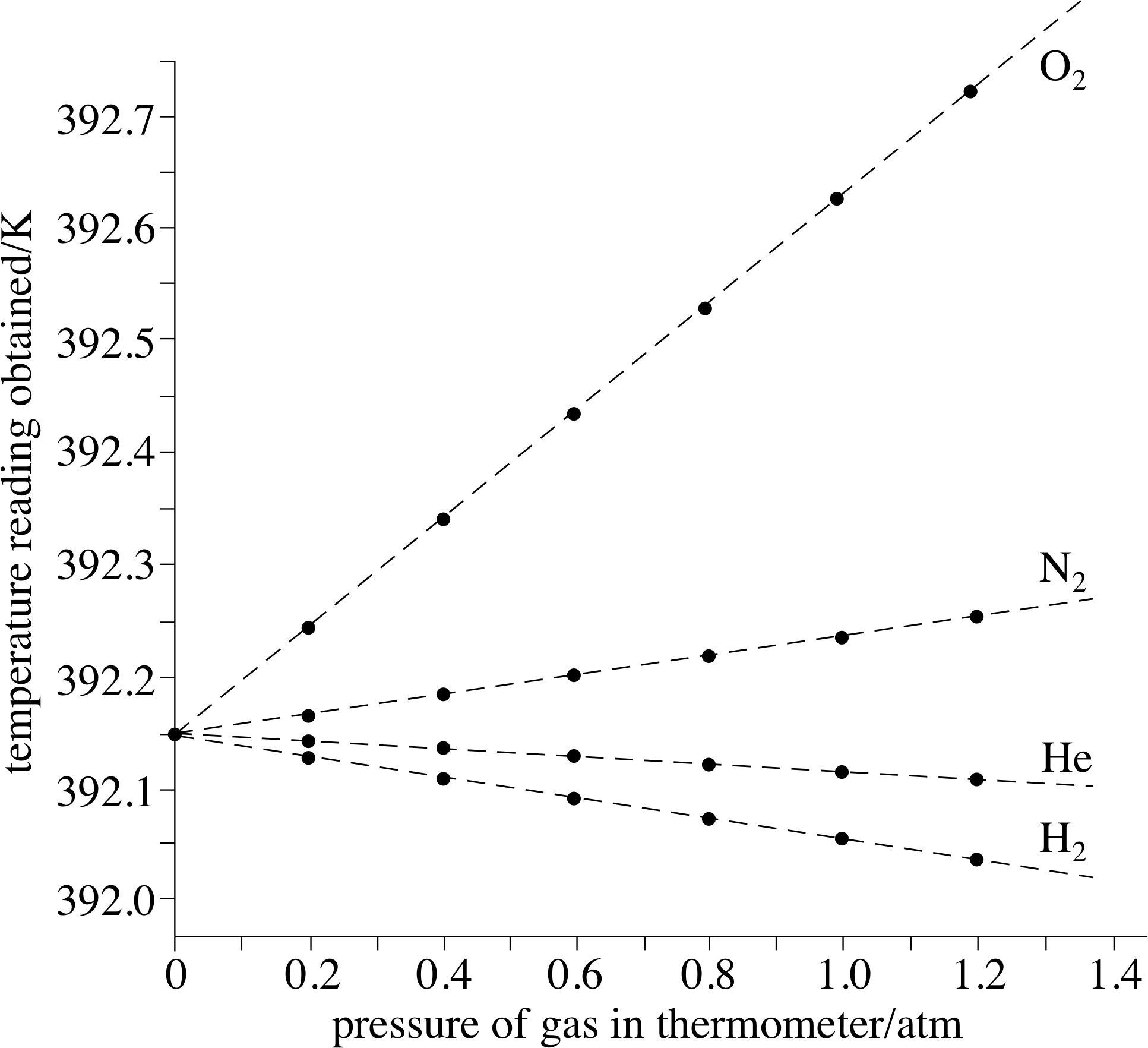
This thermometer works on the principle of Law of Gay-Lussac. The manometer is used to measure variation in pressure. What is the constant volume of gas thermometer?Ī constant volume gas thermometer ususally consists of a bulb filled with a fixed amount of a dilute gas which is attached to a mercury manometer. Does the molar volume of a gas vary with pressure?.Does the gas constant your vary with temperature?.What is the importance of gas thermometer?.What is the range of a gas thermometer?.What is the limitation of gas thermometer?.Who invented constant volume gas thermometer?.What are the advantages of constant volume gas thermometer?.Why is a constant volume gas thermometer seldom used?.How does a constant volume thermometer work?.What is the constant volume of gas thermometer?.


 0 kommentar(er)
0 kommentar(er)
